Background: PNH is an ultrarare disease characterized by complement-mediated hemolysis and subsequent anemia, bone marrow failure and thrombosis. In pts not treated with complement inhibitors, intravascular hemolysis (IVH) results from unregulated progression of the complement system and membrane attack complex formation. IVH is controlled in pts treated with anti-C5 therapy, but extravascular hemolysis (EVH) may emerge and cause persistent anemia. High LDH levels are a marker of ongoing IVH, while anemia can result from both IVH and EVH. Iptacopan is the first oral proximal complement inhibitor targeting factor B to selectively inhibit the alternative pathway of the complement cascade. As shown in PNH Phase III trials (APPLY-PNH [NCT04558918] and APPOINT-PNH [NCT04820530]), iptacopan can achieve good control of hemolysis.
Aim: To support the dose selection for iptacopan, this analysis characterizes the exposure-response relationships between iptacopan and efficacy measures (LDH and Hb) in pts with PNH. The effect of prior anti-C5 treatment status has also been evaluated.
Methods: This analysis is based on data from 4 clinical trials enrolling pts with PNH.Final data were used from the completed Phase II trials X2201 (NCT03439839; pts with active hemolysis despite anti-C5 therapy received concomitant iptacopan) and X2204 (NCT03896152; anti-C5-naïve pts received iptacopan monotherapy), in which iptacopan was administered orally in doses ranging from 25 to 200 mg twice daily (bid). Data up to the cut-off dates of 26 September 2022 and 2 November 2022 were used from the Phase III trials, APPLY-PNH (pts previously treated with anti-C5) and APPOINT-PNH (pts not treated with complement inhibitors including anti-C5), respectively, in which oral iptacopan was administered at 200 mg bid. A longitudinal mixed-effects modeling approach was applied to LDH and Hb. To account for the delay between exposure and response, indirect response models were developed for LDH and Hb. The models were used to predict the long-term (6 months) LDH and Hb responses for various iptacopan bid dose levels and to estimate (using simulation) the proportion of pts achieving LDH ≤1.5 × upper limit of normal (ULN) and Hb ≥12 g/dL.
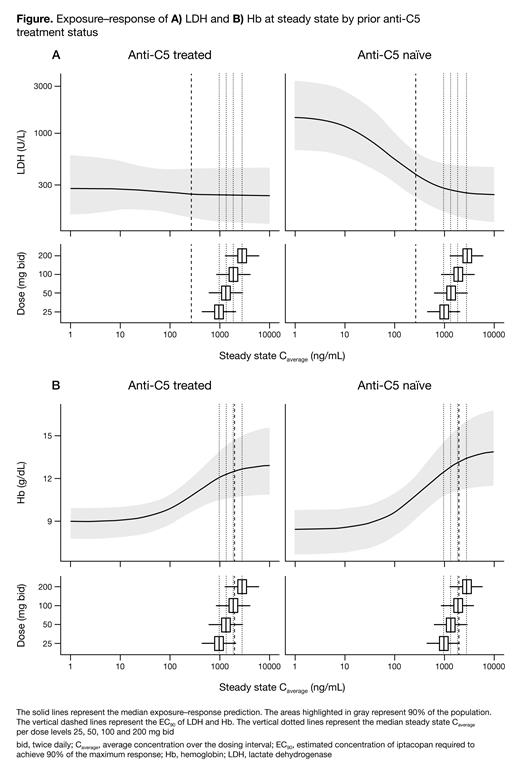
Results: The modeling dataset contained 161 pts with PNH; 94 (58%) were female, and median (range) age was 45 (18-84) years. For modeling of LDH and Hb levels, 2812 and 2587 samples were used, respectively, as well as 1577 pharmacokinetic samples. In total, 53 (33%) pts had not received anti-C5 treatment. Of the remaining 108 pts, 16 (15%) received iptacopan as add-on therapy to anti-C5 (pts from X2201) and 92 (85%) had previously received anti-C5 (pts from APPLY-PNH). The indirect response models were considered biologically plausible and adequately described the data using a common EC 50 value to both anti-C5-naïve pts and pts currently receiving or who previously received anti-C5. The estimated concentration of iptacopan required to achieve 90% of the maximum response (EC 90) of LDH was 268 ng/mL (90% confidence interval [CI] 238, 302), irrespective of prior anti-C5 treatment status ( Figure). The estimated typical LDH value after iptacopan treatment at an exposure >EC 90 was 237 U/L (90% CI 224, 250) within the normal LDH range (<250 U/L). Iptacopan 200 mg bid was the dose at which the highest proportion of pts (86%) achieved LDH ≤1.5 × ULN. While baseline LDH levels differed between anti-C5-naïve pts and pts currently receiving or who previously received anti-C5, similar proportions of these pts achieved LDH ≤1.5 × ULN (84% and 88%, respectively). The estimated EC 90 of Hb ≥12 g/dL was 1968 ng/mL (90% CI 1661, 2333) ( Figure); 89% of all pts were expected to reach EC 90 exposure at iptacopan 200 mg bid. The estimated Hb value after treatment with iptacopan at an exposure >EC 90 was 12.7 g/dL (90% CI 12.4, 13.0). 70% of pts were expected to achieve Hb normalization (≥12 g/dL) with iptacopan 200 mg bid. Similar proportions of anti-C5-naïve pts and pts who were currently receiving or who previously received anti-C5 achieved Hb ≥12 g/dL (71% and 68%, respectively).
Conclusions: Indirect response models were developed to describe the exposure-response relationships between iptacopan and efficacy measures (LDH and Hb) in pts with PNH. The analysis demonstrated that 200 mg bid is the dose of oral iptacopan that achieves LDH ≤1.5 × ULN and Hb normalization in the highest proportion of patients regardless of prior treatment status.
Disclosures
Risitano:Novartis: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Research Funding. Peffault De Latour:Jazz Pharmaceuticals: Honoraria. Bartels:Novartis Pharma AG: Current Employment, Current equity holder in publicly-traded company. Junge:Novartis Institutes of Biomedical Research: Current Employment, Current equity holder in publicly-traded company. Dahlke:Novartis Pharma AG: Current Employment. Baltcheva:Novartis Pharma AG: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal